Publications
Cell Reports - Adipocyte mesenchymal transition contributes to mammary tumor progression
Zhu Q, ZhuY, Hepler C, Zhang Q, Park J, Gliniak J Henry GH, Crewe C, Bu D, Zhang Z, Zhao S, Morley T, Li N, Kim D, Strand D, Deng Y, Robino JJ, Varlamov O, Gordillo R,Kolonin MG, Kusminski CM, Gupta RK, Scherer PE
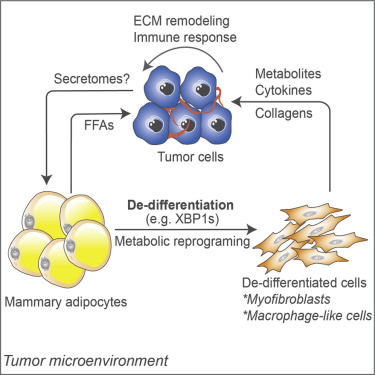
Obesity is associated with increased cancer incidence and progression. However, the relationship between adiposity and cancer remains poorly understood at the mechanistic level. Here, we report that adipocytes from tumor-invasive mammary fat undergo de-differentiation to fibroblast-like precursor cells during tumor progression and integrate into the tumor microenvironment. Single-cell sequencing reveals that these de-differentiated adipocytes lose their original identities and transform into multiple cell types, including myofibroblast- and macrophage-like cells, with their characteristic features involved in immune response, inflammation, and extracellular matrix remodeling. The de-differentiated cells are metabolically distinct from tumor-associated fibroblasts but exhibit comparable effects on tumor cell proliferation. Inducing de-differentiation by Xbp1s overexpression promotes tumor progression despite lower adiposity. In contrast, promoting lipid-storage capacity in adipocytes through MitoNEET overexpression curbs tumor growth despite greater adiposity. Collectively, the metabolic interplay between tumor cells and adipocytes induces adipocyte mesenchymal transition and contributes to reconfigure the stroma into a more tumor-friendly microenvironment.
September 2022
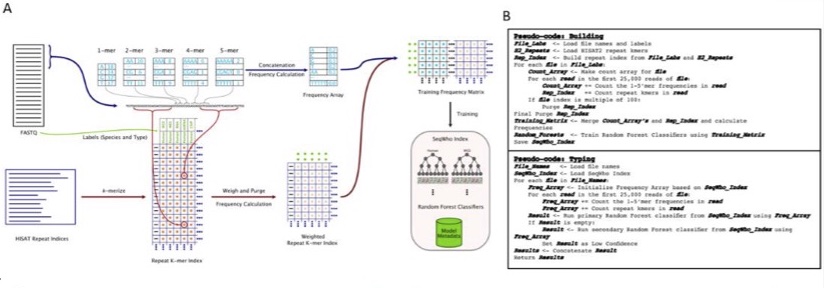
Bioinformatics - SeqWho: reliable, rapid determination of sequence file identity using k-mer frequencies in Random Forest classifiers
Bennett C, Thornton M, Park C, Henry G, Zhang Y, Malladi V, Kim D
With the vast improvements in sequencing technologies and increased number of protocols, sequencing is being used to answer complex biological problems. Subsequently, analysis pipelines have become more time consuming and complicated, usually requiring highly extensive prevalidation steps. Here, we present SeqWho, a program designed to assess heuristically the quality of sequencing files and reliably classify the organism and protocol type by using Random Forest classifiers trained on biases native in k-mer frequencies and repeat sequence identities. Results Using one of our primary models, we show that our method accurately and rapidly classifies human and mouse sequences from nine different sequencing libraries by species, library and both together, 98.32%, 97.86% and 96.38% of the time, respectively. Ultimately, we demonstrate that SeqWho is a powerful method for reliably validating the quality and identity of the sequencing files used in any pipeline
February 2022
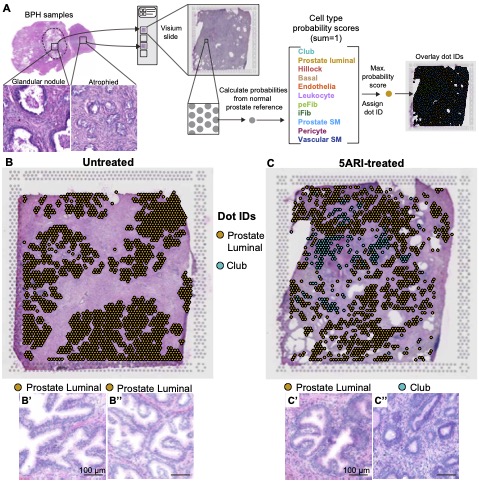
The Journal of Pathology - 5-alpha reductase inhibitors induce a prostate luminal to club cell transition in human benign prostatic hyperplasia
Joseph DB, Henry GH, Malewska A, Reese JC, Mauck RJ, Gahan JC, Hutchinson RC, Mohler JL, Roehrborn CG, Strand DW
Benign prostatic hyperplasia (BPH) is a non-malignant expansion of peri-urethral prostate tissue that affects half of men by age 50aging men. Patients with enlarged prostates are treated with 5-alpha reductase inhibitors (5ARIs) are used as a frontline therapy for BPH andto shrink prostate volume by blocking the conversion of testosterone to dihydrotestosterone (DHT). Low DHT levels elicit apoptosis of prostate secretory luminal cells and epithelial atrophyluminal epithelial apoptosis, but the histologic response to 5ARI treatment is often heterogeneous and lower urinary tract symptoms often persist, requiring surgical intervention. We used two spatial transcriptomics profiling approaches to characterize gene expression changes across histologically normal and atrophied regions in prostates from 5ARI-treated men. OUsing objective profiling with using the Visium spatial gene expression platform, we foundshowed that atrophied acini from 5ARI treated patients with decreased epithelial infolding and reduced lumen size were high in a club-cell gene signature in 5ARI treated patientshad increased club cell gene expression. The spectrum of morphological atrophy changes in prostate acini from 5ARI treated patients ending with atrophy correlated with reduced androgen receptor signaling and increased expression of urethral club cell genes including LTF, PIGR, OLFM4, SCGB1A1 and SCGB3A1. Prostate luminal cells within atrophied acini also adapted to low DHT conditions by increasing NF-κB signaling and anti-apoptotic BCL2 expression, which may explain their survival. Using nanoString GeoMx digital spatial profiling, we confirmed that histologically atrophied acini with expressing SCGB3A1 expression displayed higher levels of club cell markers compared to histologically normal, NKX3-1 expressing+ regions that retained prostate identity. In addition, club-like cells within regions of 5ARI induced prostate atrophy closely resembled club cells from the urethral epithelium. A comparison of histologically normal regions from untreated men and histologically normal regions from 5ARI-treated men revealed only few transcriptional differences, highlighting the mixed response to 5ARI treatment where some acini are unaffected. Our Together, our results describe a heterogeneous response to 5ARI treatment where some epithelial regions undergo an epithelial adaptation of from a prostate secretory luminal cells to a club cell-like state in response to 5ARI treatment.
December 2021
The Journal of Pathology - Single cell analysis of mouse and human prostate reveals novel fibroblasts with specialized distribution and microenvironment interactions
Joseph DB, Henry GH, Malewska A, Reese JC, Mauck RJ, Gahan JC, Hutchinson RC, Malladi VS, Roehrborn CG, Vezina CM, Strand DW
Stromal-epithelial interactions are critical to the morphogenesis, differentiation and homeostasis of the prostate, but the molecular identity and anatomy of discrete stromal cell types is poorly understood. Using single cell RNA-sequencing, we identified and validated the in situ localization of three smooth muscle subtypes (prostate smooth muscle, pericytes and vascular smooth muscle) and two novel fibroblast subtypes in human prostate. Peri-epithelial fibroblasts (APOD+) wrap around epithelial structures while interstitial fibroblasts (C7+) are interspersed in extracellular matrix. In contrast, the mouse displayed three fibroblast subtypes with distinct proximal-distal and lobe specific distribution patterns. Statistical analysis of mouse and human fibroblasts showed transcriptional correlation between mouse prostate (C3+) and urethral (Lgr5+) fibroblasts and the human interstitial fibroblast subtype. Both urethral fibroblasts (Lgr5+) and ductal fibroblasts (Wnt2+) in the mouse contribute to a proximal Wnt/Tgfb signaling niche that is absent in human prostate. Instead, human peri-epithelial fibroblasts express secreted WNT inhibitors SFRPs and DKK1, which could serve as a buffer against stromal WNT ligands by creating a localized signaling niche around individual prostate glands. We also identified proximal-distal fibroblast density differences in human prostate that could amplify stromal signaling around proximal prostate ducts. In human Benign Prostatic Hyperplasia, fibroblast subtypes upregulate critical immunoregulatory pathways and show distinct distributions in stromal and glandular phenotypes. A detailed taxonomy of leukocytes in BPH reveals an influx of myeloid dendritic cells, T cells and B cells, resembling a mucosal inflammatory disorder. A receptor-ligand interaction analysis of all cell types revealed a central role for fibroblasts in growth factor, morphogen and chemokine signaling to endothelia, epithelia and leukocytes. These data are foundational to the development of new therapeutic targets in benign prostatic hyperplasia.
June 2021
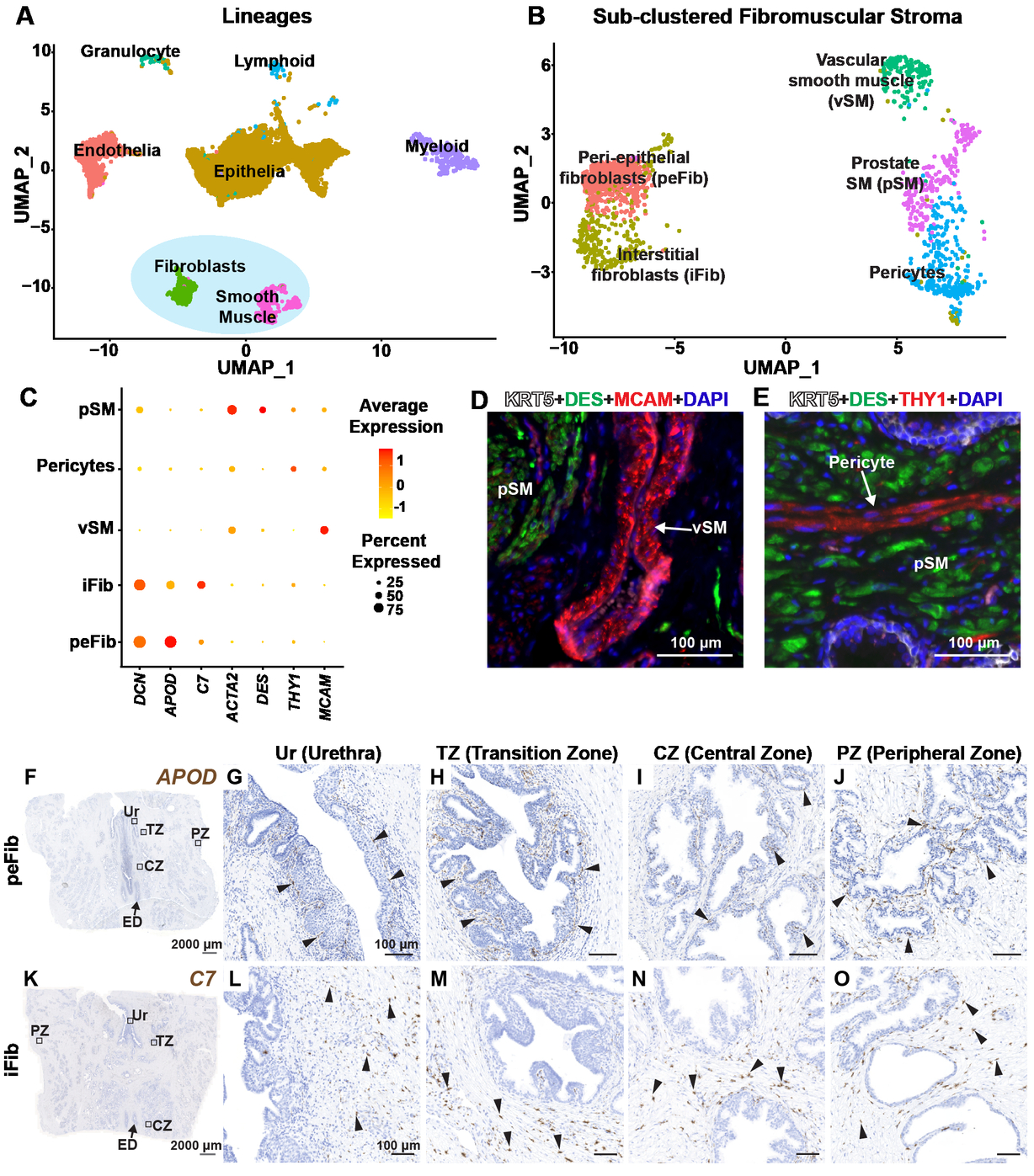
Physiological Reports - A NEW Approach for Characterizing Mouse Urinary Pathophysiologies
Ruetten H, Henry G, Liu T, Spratt H, Ricke W, Strand D, Vezina C
The void spot assay (VSA) is a cost-effective method for evaluating and quantifying mouse urinary voiding phenotypes. The VSA has been used to differentiate voiding behaviors between experimental groups, but not as a diagnostic assay. To build towards this goal, we used the VSA to define voiding patterns of male mice with diabetic diuresis (BTBR.Cg-Lepob /WiscJ mice), irritative urinary dysfunction (E. coli UTI89 urinary tract infection), and obstructive urinary dysfunction (testosterone and estradiol slow-release implants) compared to their respective controls. Many studies compare individual VSA endpoints (urine spot size, quantity or distribution) between experimental groups. Here, we consider all endpoints collectively to establish VSA phenomes of mice with three different etiologies of voiding dysfunction. We created an approach called Normalized Endpoint Work Through (NEW) to normalize VSA outputs to control mice, and then applied Principal Components Analysis and hierarchical clustering to 12 equally weighted, normalized, scaled, and zero-centered VSA outcomes collected from each mouse (the VSA phenome). This approach accurately classifies mice based on voiding dysfunction etiology. We used principal components analysis and hierarchical clustering to show that some aged mice (>24 m old) develop an obstructive or a diabetic diuresis VSA phenotype while others develop a unique phenotype that does not cluster with that of diabetic, infected, or obstructed mice. These findings support use of the VSA to identify specific urinary phenotypes in mice and the continued use of aged mice as they develop urinary dysfunction representative of the various etiologies of LUTS in men.
June 2021
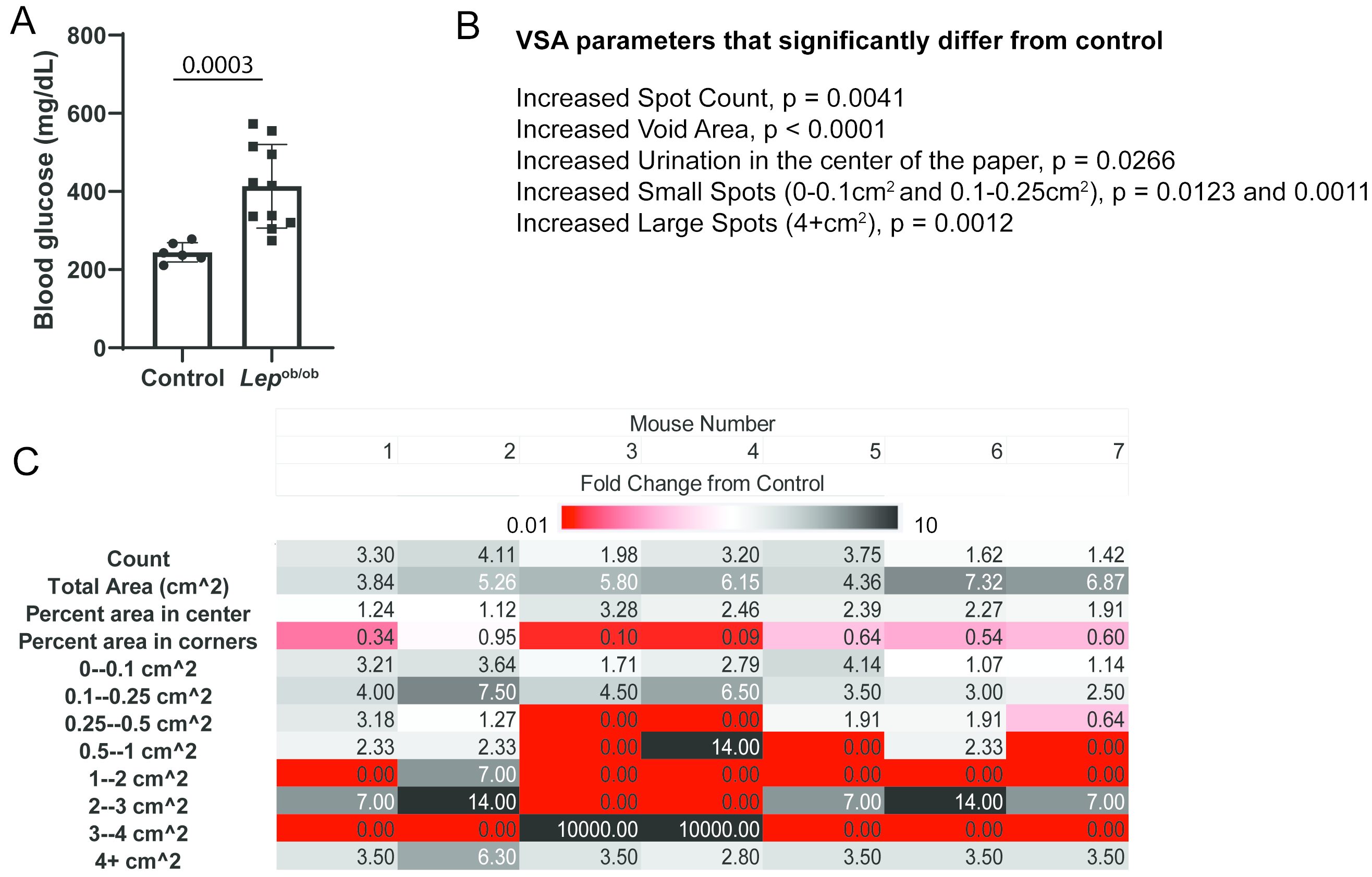
Cell Stem Cell - Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity
Shao M, Hepler C, Zhang Q, Shan B, Vishvanath L, Henry GH, Zhao S, An YA, Wu Y, Strand DW, Gupta RK
Adipose precursor cells (APCs) exhibit regional variation in response to obesity, for unclear reasons. Here, we reveal that HIFα-induced PDGFRβ signaling within murine white adipose tissue (WAT) PDGFRβ+ cells drives inhibitory serine 112 (S112) phosphorylation of PPARγ, the master regulator of adipogenesis. Levels of PPARγ S112 phosphorylation in WAT PDGFRβ+ cells are depot dependent, with levels of PPARγ phosphorylation in PDGFRβ+ cells inversely correlating with their capacity for adipogenesis upon high-fat-diet feeding. HIFα suppression in PDGFRβ+ progenitors promotes subcutaneous and intra-abdominal adipogenesis, healthy WAT remodeling, and improved metabolic health in obesity. These metabolic benefits are mimicked by treatment of obese mice with the PDGFR antagonist Imatinib, which promotes adipocyte hyperplasia and glucose tolerance in a progenitor cell PPARγ-dependent manner. Our studies unveil a mechanism underlying depot-specific responses of APCs to high-fat feeding and highlight the potential for APCs to be targeted pharmacologically to improve metabolic health in obesity.
February 2021
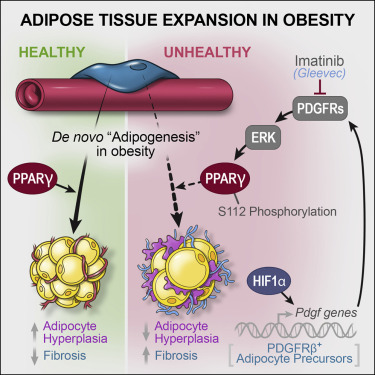
The Prostate - Urethral luminal epithelia are castration-insensitive cells of the proximal prostate
Joseph DB, Henry GH, Malewska A, Iqbal NS, Ruetten HM, Turco AE, Abler LL, Sandhu SK, Cadena MK, Malladi VS, Reese JC, Mauck RJ, Gahan JC, Hutchinson RC, Roehrborn CG, Baker LA, Vezina CM, Strand DW
Castration‐insensitive epithelial progenitors capable of regenerating the prostate have been proposed to be concentrated in the proximal region based on facultative assays. Functional characterization of prostate epithelial populations isolated with individual cell surface markers has failed to provide a consensus on the anatomical and transcriptional identity of proximal prostate progenitors. Here, we use single‐cell RNA sequencing to obtain a complete transcriptomic profile of all epithelial cells in the mouse prostate and urethra to objectively identify cellular subtypes. Pan‐transcriptomic comparison to human prostate cell types identified a mouse equivalent of human urethral luminal cells, which highly expressed putative prostate progenitor markers. Validation of the urethral luminal cell cluster was performed using immunostaining and flow cytometry. Our data reveal that previously identified facultative progenitors marked by Trop2, Sca-1, KRT4, and PSCA are actually luminal epithelial cells of the urethra that extend into the proximal region of the prostate, and are resistant to castration‐induced androgen deprivation. Mouse urethral luminal cells were identified to be the equivalent of previously identified human club and hillock cells that similarly extend into proximal prostate ducts. Benign prostatic hyperplasia (BPH) has long been considered an “embryonic reawakening,” but the cellular origin of the hyperplastic growth concentrated in the periurethral region is unclear. We demonstrate an increase in urethral luminal cells within glandular nodules from BPH patients. Urethral luminal cells are further increased in patients treated with a 5‐α reductase inhibitor. Our data demonstrate that cells of the proximal prostate that express putative progenitor markers, and are enriched by castration in the proximal prostate, are urethral luminal cells and that these cells may play an important role in the etiology of human BPH.
June 2020
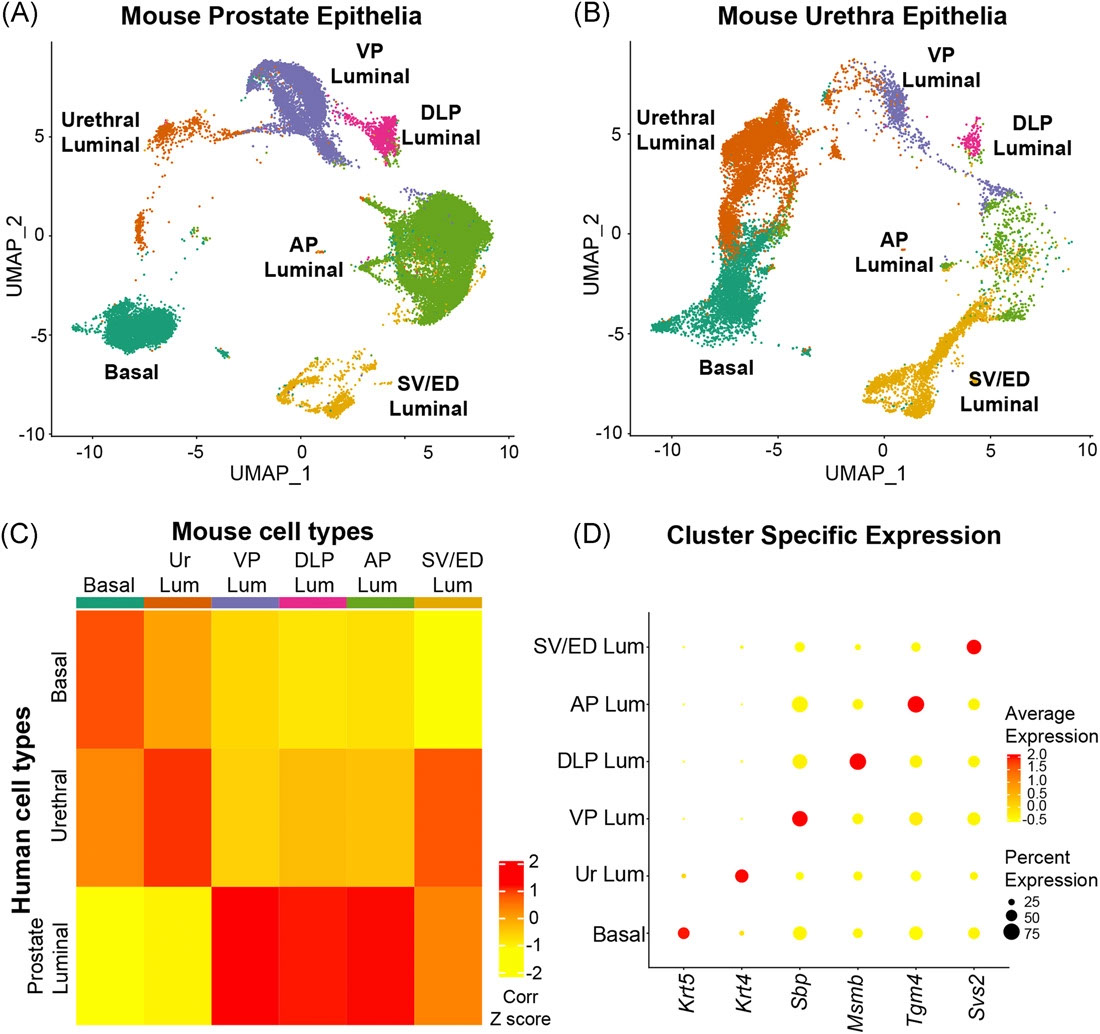
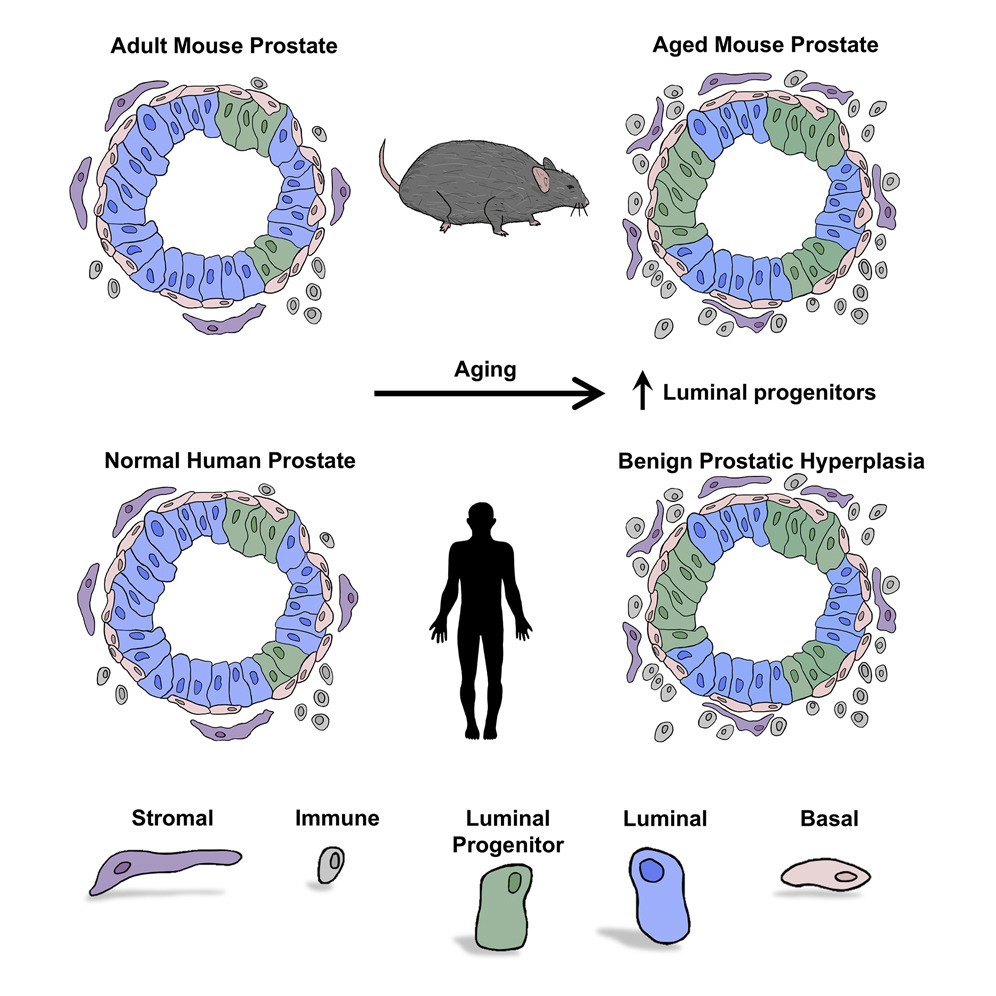
Cell Reports - Expansion of Luminal Progenitor Cells in the Aging Mouse and Human Prostate
Crowell PD, Fox JJ, Hashimoto T, Diaz JA, Havarro HI, Henry GH, Feldmar BA, Lowe MG, Garcia AJ, Wu YE, Sajed DP, Strand DW, Goldstein AS
Aging is associated with loss of tissue mass and a decline in adult stem cell function in many tissues. In contrast, aging in the prostate is associated with growth-related diseases including benign prostatic hyperplasia (BPH). Surprisingly, the effects of aging on prostate epithelial cells have not been established. Here we find that organoid-forming progenitor activity of mouse prostate basal and luminal cells is maintained with age. This is caused by an age-related expansion of progenitor-like luminal cells that share features with human prostate luminal progenitor cells. The increase in luminal progenitor cells may contribute to greater risk for growth-related disease in the aging prostate. Importantly, we demonstrate expansion of human luminal progenitor cells in BPH. In summary, we define a Trop2+ luminal progenitor subset and identify an age-related shift in the luminal compartment of the mouse and human prostate epithelium.
August 2019
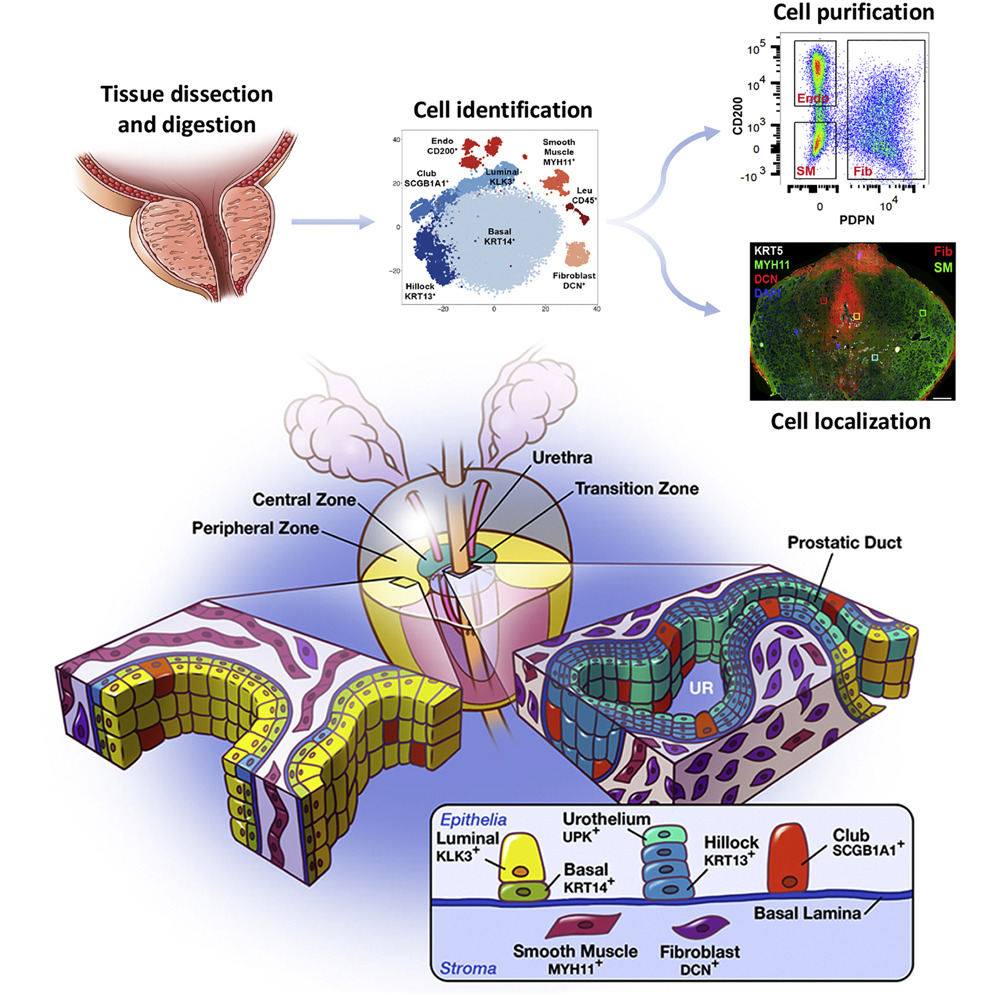
Cell Reports - A cellular anatomy of the normal human prostate
Henry GH, Malewska A, Joseph DB, Malladi VS, Lee J, Torrealba J, Mauck RJ, Gahan JC, Raj GV, Roehrborn CG, Hon GC, MacConmara MP, Reese JC, Hutchinson RC, Vezina CM, Strand DW
A comprehensive cellular anatomy of normal human prostate is essential for solving the cellular origins of benign prostatic hyperplasia and prostate cancer. The tools used to analyze the contribution of individual cell types are not robust. We provide a cellular atlas of the young adult human prostate and prostatic urethra using an iterative process of single-cell RNA sequencing (scRNA-seq) and flow cytometry on ∼98,000 cells taken from different anatomical regions. Immunohistochemistry with newly derived cell type-specific markers revealed the distribution of each epithelial and stromal cell type on whole mounts, revising our understanding of zonal anatomy. Based on discovered cell surface markers, flow cytometry antibody panels were designed to improve the purification of each cell type, with each gate confirmed by scRNA-seq. The molecular classification, anatomical distribution, and purification tools for each cell type in the human prostate create a powerful resource for experimental design in human prostate disease.
December 2018
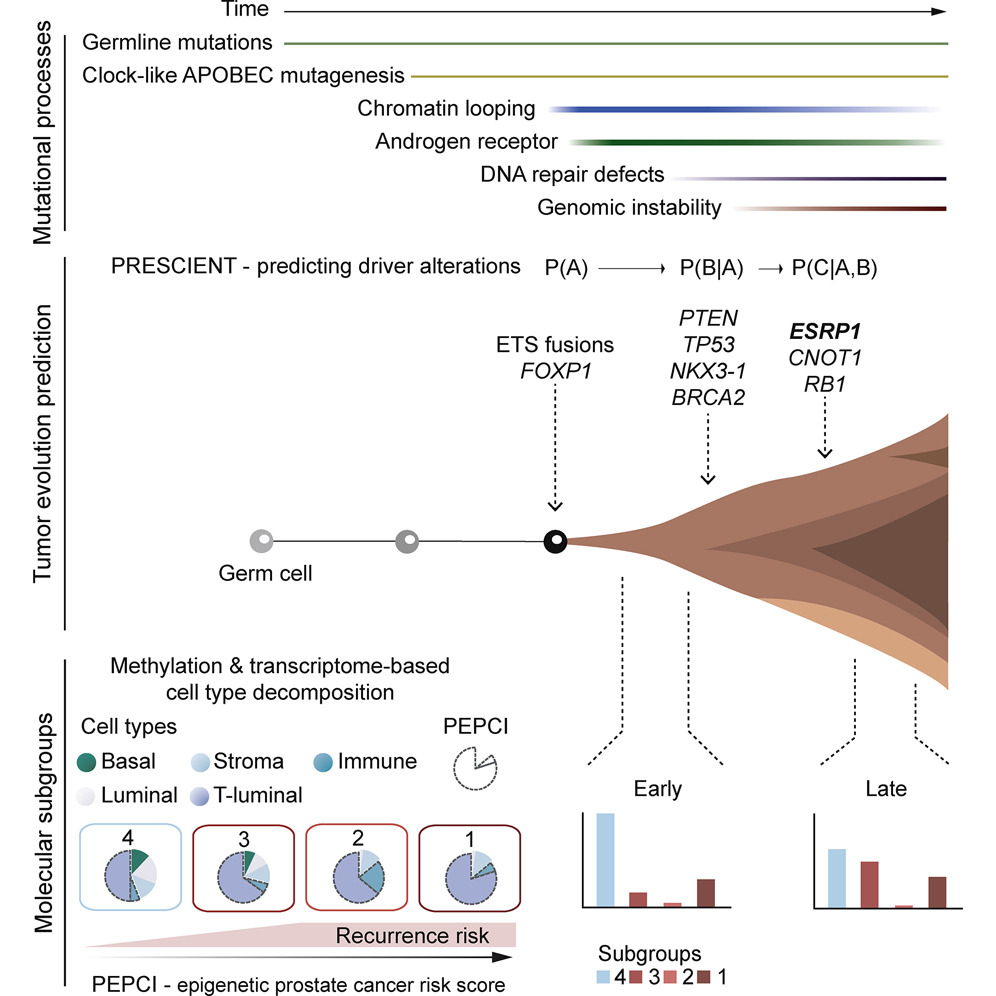
Cancer Cell - Molecular evolution of early-onset prostate cancer identifies molecular risk markers and clinical trajectories
Gerhauser C, Favero F, Risch T, Simon R, Feuerbach L, Assenov Y, Heckmann D, Sidiropoulos N, Waszak SM, Hubschmann D, Urbanucci A, Girma EG, Kuryshev V, Klimczak LJ, Saini N, Stutz AM, Weichenhan D, Bottcher LM, Toth R, Hendriksen JD, Koop C, Lutsik P, Matzk S, Warnatz HJ, Amstislavskiy V, Feuerstein C, Raeder B, Bogatyrova O, Schmitz EM, Hube-Magg C, Kluth M, Huland H, Graefen M, Lawerenz C, Henry GH, Yamaguchi TN, Malewska A, Meiners J, Schilling D, Reisinger E, Eils R, Schlesner M, Strand DW, Bristow RG, Boutros PC, von Kalle C, Gordenin D, Sultmann H, Brors B, Sauter G, Plass C, Yaspo ML, Korbel JO, Schlomm T, Weischenfeldt J
Identifying the earliest somatic changes in prostate cancer can give important insights into tumor evolution and aids in stratifying high- from low-risk disease. We integrated whole genome, transcriptome and methylome analysis of early-onset prostate cancers (diagnosis ≤55 years). Characterization across 292 prostate cancer genomes revealed age-related genomic alterations and a clock-like enzymatic-driven mutational process contributing to the earliest mutations in prostate cancer patients. Our integrative analysis identified four molecular subgroups, including a particularly aggressive subgroup with recurrent duplications associated with increased expression of ESRP1, which we validate in 12,000 tissue microarray tumors. Finally, we combined the patterns of molecular co-occurrence and risk-based subgroup information to deconvolve the molecular and clinical trajectories of prostate cancer from single patient samples.
December 2018
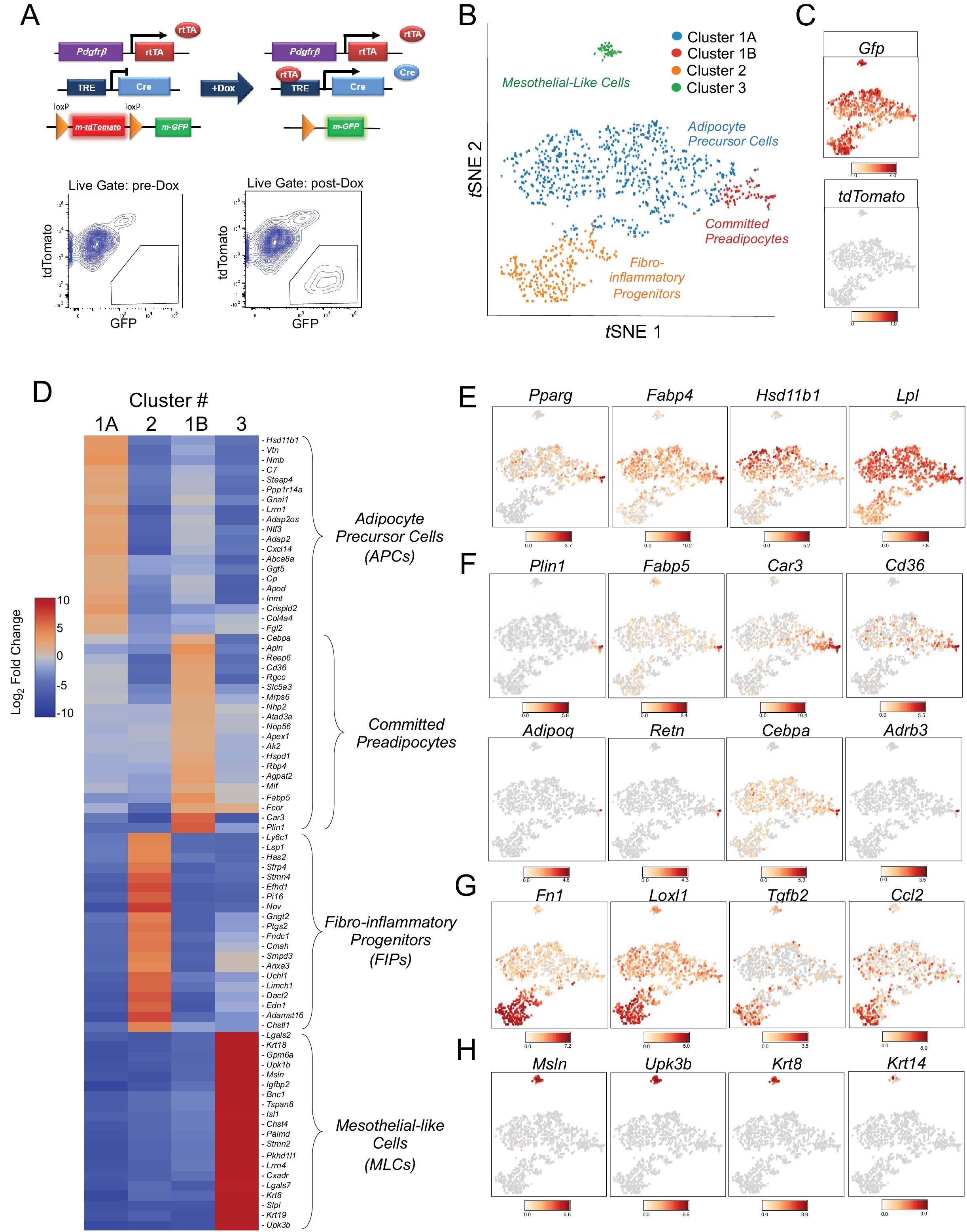
eLife - Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice
Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand DW, Hon GC, Gupta RK
White adipose tissue (WAT) remodeling is dictated by coordinated interactions between adipocytes and resident stromal-vascular cells; however, the functional heterogeneity of adipose stromal cells has remained unresolved. We combined single-cell RNA-sequencing and FACS to identify and isolate functionally distinct subpopulations of PDGFRβ+ stromal cells within visceral WAT of adult mice. LY6C- CD9- PDGFRβ+ cells represent highly adipogenic visceral adipocyte precursor cells (‘APCs’), whereas LY6C+ PDGFRβ+ cells represent fibro-inflammatory progenitors (‘FIPs’). FIPs lack adipogenic capacity, display pro-fibrogenic/pro-inflammatory phenotypes, and can exert an anti-adipogenic effect on APCs. The pro-inflammatory phenotype of PDGFRβ+ cells is regulated, at least in part, by NR4A nuclear receptors. These data highlight the functional heterogeneity of visceral WAT perivascular cells, and provide insight into potential cell-cell interactions impacting adipogenesis and inflammation. These improved strategies to isolate FIPs and APCs from visceral WAT will facilitate the study of physiological WAT remodeling and mechanisms leading to metabolic dysfunction.
September 2018
Cytometry Part A - OMIP-040: Optimized gating of human prostate cellular subpopulations
Henry GH, Loof N, Strand DW
This panel was optimized to quantify the relative frequency of the major cell types present in the human prostate in addition to their sorting for downstream applications. Tissue resident white blood cells (referred to here as leukocytes) are identified by CD45, epithelia are identified by CD326, and stroma are double negative with those markers. Epithelia can be further segregated into basal, luminal, and “other” populations using CD26 and CD271. Fibromuscular stroma can be identified by removing CD31‐positive endothelia from the stroma. This panel can also serve as a backbone for the addition of markers to interrogate subpopulations within these major cell types. The panel has been validated on freshly digested and cryopreserved human prostate cells collected from young organ donors, BPH patients, and prostate cancer patients. Other tissue types have not been tested. Other basal cell markers including CD49f, podoplanin, and CD104 are tested and compared with CD271.
August 2017
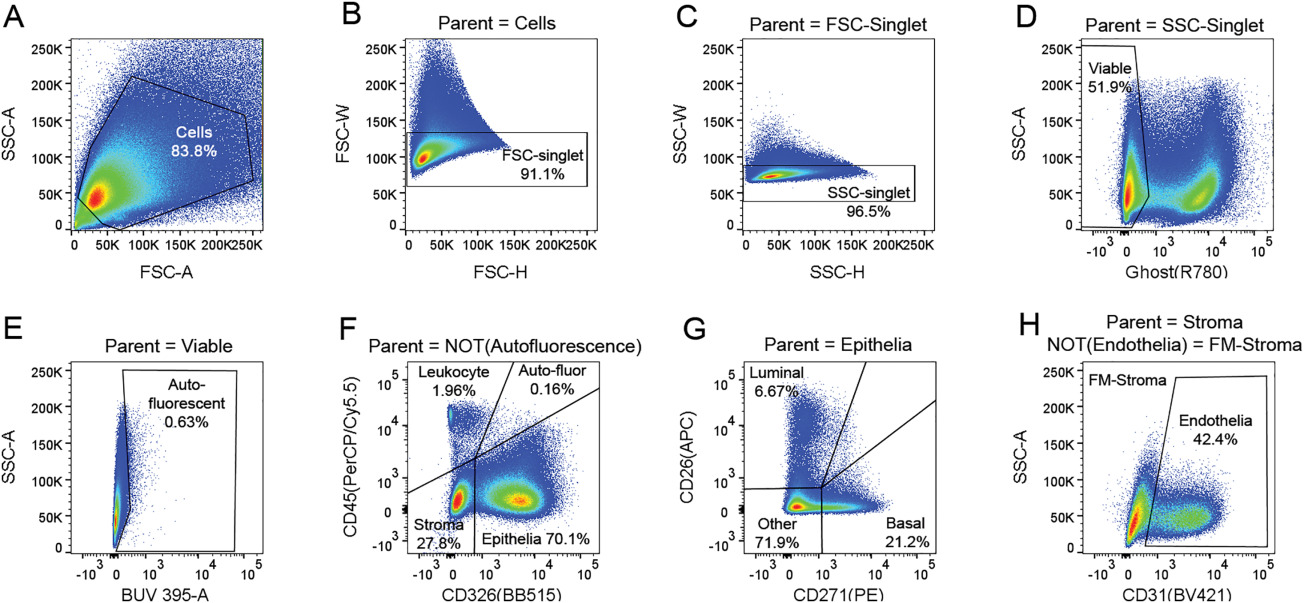
The Prostate - Molecular pathogenesis of human prostate basal cell hyperplasia
Henry GH, Malewska A, Hutchinson R, Gahan J, Mauck R, Francis F, Torrealba J, Roehrborn C, Strand DW
Background: Understanding the molecular pathogenesis of distinct phenotypes in human benign prostatic hyperplasia (BPH) is essential to improving therapeutic intervention. Current therapies target smooth muscle and luminal epithelia for relief of lower urinary tract symptoms (LUTS) due to BPH, but basal cell hyperplasia (BCH) remains untargeted. The incidence of has been reported at 8‐10%, but a molecular and cellular characterization has not been performed on this phenotype. Methods: Using freshly digested tissue from surgical specimens, we performed RNA‐seq analysis of flow cytometry‐purified basal epithelia from 3 patients with and 4 patients without a majority BCH phenotype. qPCR was performed on 28 genes identified as significant from 13 non‐BCH and 7 BCH specimens to confirm transcriptomic analysis. IHC was performed on several non‐BCH and BCH specimens for 3 proteins identified as significant by transcriptomic analysis. Results: A total of 141 human BPH specimens were analyzed for the presence of BCH. Clinical characteristics of non‐BCH and BCH cohorts revealed no significant differences in age, PSA, prostate volume, medical treatment, or comorbidities. Quantitation of cellular subsets by flow cytometry in 11 BCH patients vs. 11 non‐BCH patients demonstrated a significant increase in the ratio of basal to luminal epithelia in patients with BCH (P <0.05), but no significant differences in the total number of leukocytes. RNA‐seq data from flow cytometry isolated basal epithelia from patients with and without BCH were subjected to gene set enrichment analysis of differentially expressed genes, which revealed increased expression of members of the epidermal differentiation complex. Transcriptomic data were complemented by immunohistochemistry for members of the epidermal differentiation complex, revealing a morphological similarity to other stratified squamous epithelial layers. Conclusions: Increased expression of epidermal differentiation complex members and altered epithelial stratification resembles the progression of other metaplastic diseases. These data provide insight into the plasticity of the human prostate epithelium and suggest a classification of basal cell hyperplasia as a metaplasia.
June 2017
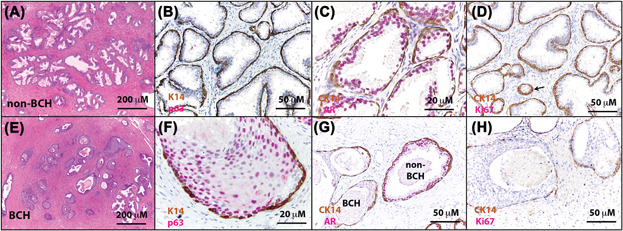
Molecular Cell - Non-cell-autonomous regulation of prostate epithelial homeostasis by androgen receptor
Zhang B, Kwon OJ, Henry G, Malewska A, Wei X, Zhang L, Brinkley W, Zhang Y, Castro PD, Titus M, Chen R, Sayeeduddin M, Raj GV, Mauck R, Roehrborn C, Creighton CJ, Strand DW, Ittmann MM, Xin L
Prostate inflammation has been suggested as an etiology for benign prostatic hyperplasia (BPH). We show that decreased expression of the androgen receptor (AR) in luminal cells of human BPH specimens correlates with a higher degree of regional prostatic inflammation. However, the cause-and-effect relationship between the two events remains unclear. We investigated specifically whether attenuating AR activity in prostate luminal cells induces inflammation. Disrupting luminal cell AR signaling in mouse models promotes cytokine production cell-autonomously, impairs epithelial barrier function, and induces immune cell infiltration, which further augments local production of cytokines and chemokines including Il-1 and Ccl2. This inflammatory microenvironment promotes AR-independent prostatic epithelial proliferation, which can be abolished by ablating IL-1 signaling or depleting its major cellular source, the macrophages. This study demonstrates that disrupting luminal AR signaling promotes prostate inflammation, which may serve as a mechanism for resistance to androgen-targeted therapy for prostate-related diseases.
September 2016
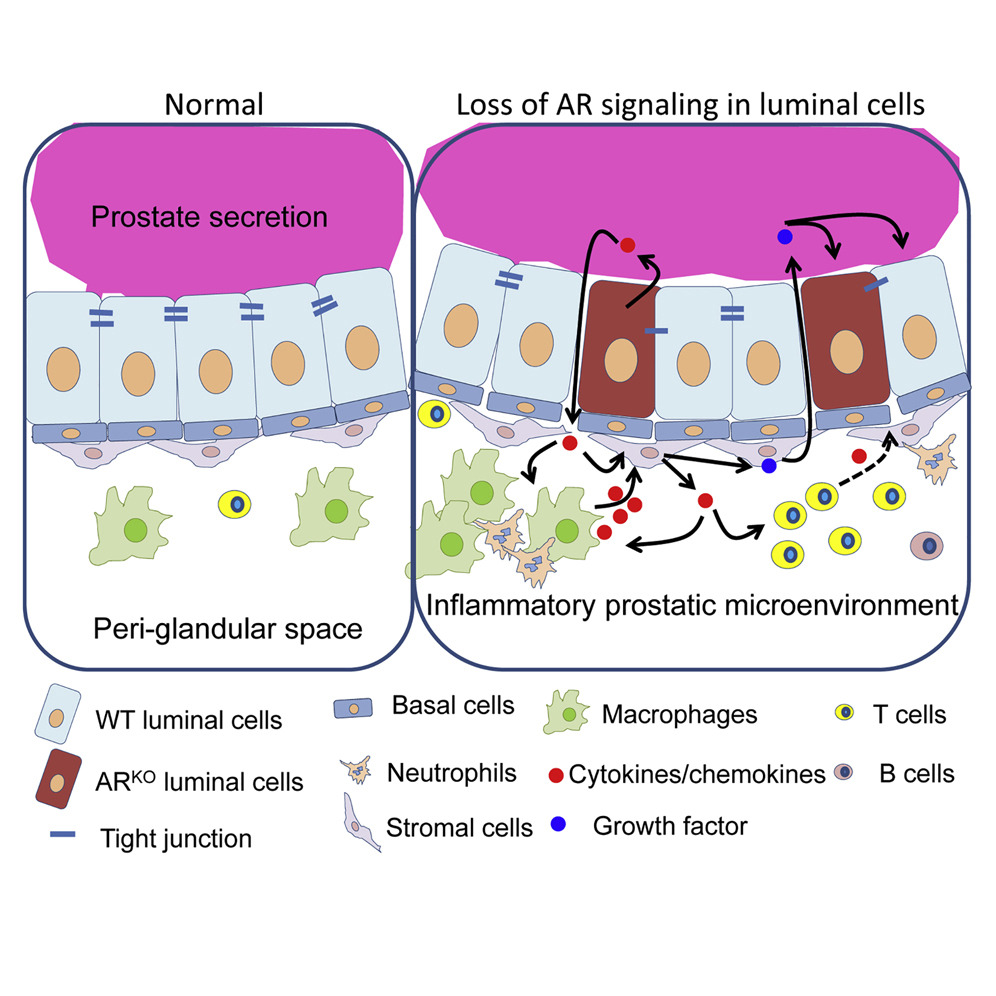
Differentiation - Isolation and analysis of discrete human prostate cellular populations
Strand D W, Aaron L, Henry G, Franco O E, Hayward SW
The use of lineage tracing in transgenic mouse models has revealed an abundance of subcellular phenotypes responsible for maintaining prostate homeostasis. The ability to use fresh human tissues to examine the hypotheses generated by these mouse experiments has been greatly enhanced by technical advances in tissue processing, flow cytometry and cell culture. We describe in detail the optimization of protocols for each of these areas to facilitate research on solving human prostate diseases through the analysis of human tissue.
April 2016
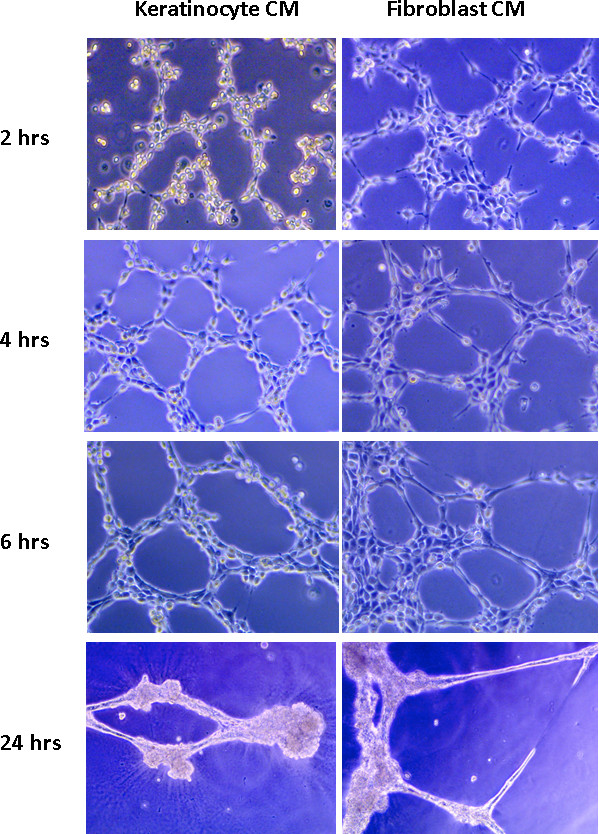
JoVE - Endothelial cell tube formation assay for the in vitro study of angiogenesis
DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L, O’Neill RC, Morin A, Wiest JS
The tube formation assay is a fast, quantifiable method for measuring in vitro angiogenesis. Endothelial cells are combined with conditioned media and plated on basement membrane extract. Tube formation occurs within hours and newly formed tubules easily quantified.
September 2014